Periodic Table Element Comparison: Tin and Lead
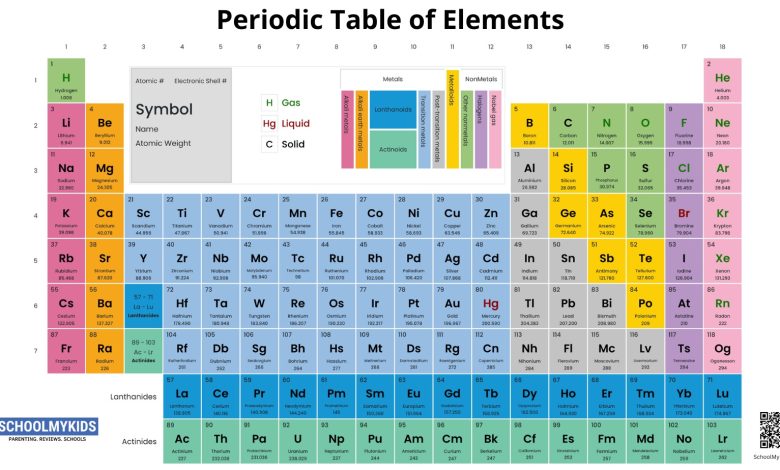
The periodic table is packed with practical information that helps us understand the elements, which are the building blocks of the universe. The 118 elements known to us today are organised in the periodic table. The horizontal rows of the periodic table are known as periods, while the vertical columns are known as groups. The elements in each group share similar characteristics.
Counting from left to right, the carbon group is the fourteenth column and is sometimes called group 14. There are five elements in the group carbon at the top, then silicon, germanium, tin and lead. Each element is given a one- or two-letter symbol on the table. Carbon’s symbol is C; silicon’s is Si; germanium’s is Ge, tin’s is Sn, and lead is Pb.
Tin’s and lead’s symbols may seem peculiar at first glance. This is because their symbols are not based on their English names. Many years ago, chemists used the Latin names of tin and lead to create their symbols. In Latin, tin is known as stannum, while lead is plumbum. The elements of the carbon group all have numerous functions, and each is important in its own unique way.
Similarities between Tin and Lead
The following points show the similarities between the two elements.
- Although both the elements have electropositive character, yet, being in the middle of the periodic table, they also show feeble electronegative character in some of their compounds like halides, metastannates, metaplumbates, etc.
- Both elements form highly unstable hydrides.
- They form quite stable halides. The formation of such halides indicates that these elements have feeble electronegative character.
- Both elements form similar complexes, namely stannates and plumbates, respectively.
- When caustic alkali is added to an Sn(+2) salt solution in the absence of air, Sn(OH)2 is first precipitated that dissolves in excess of alkali, forming stannite (SnO2). Similarly, a plumbite (PbO2) is formed by the addition of an excess of alkali to a Pb (+2) salt solution.
- Metastannates (e.g., Na2SnO3) and metaplumbates (e.g., NaPbO3) are stable and well-defined compounds.
General Properties of Carbon Group Elements
Each element in the periodic table can be described by its properties. There are two different types of properties-chemical and physical. Chemical properties have to do with how an element reacts with other elements. Some elements are very reactive; others, not so much. When elements react, they may produce heat, explode, glow or change colours. The colourful display of a firework show is a result of chemical reactions, as is the burning of jet fuel that pushes an aeroplane through the air.
Chemical reactions do not always need to be explosive, colourful or even visible. For example, in each leaf of a plant, there are thousands of chemical reactions occurring at any given time. It is not noticed because they are not very energetic and are hidden within the leaf. Over time, these reactions result in noticeable changes, such as the growth of the leaf or changes in the leaf’s colour.
Chemical reactions occur around us all the time and are essential to life on our planet. The elements of the carbon group – carbon, silicon, germanium, tin and lead, have similar chemical properties, which is why they are grouped together in the same column of the periodic table. For example, the elements of the carbon group all react with oxygen to form chemical compounds called oxides. A compound is made of two or more elements held together by electrical forces. These forces are known as chemical bonds.





