What are the prerequisites for chemical kinetics and solutions?
Although all the chapters in chemistry are important in achieving good grades in normal school examinations, board exams, and national level competitive exams like as NEET and JEE mains. However, it has been noted that the majority of students are uncomfortable with several aspects of this subject. Though it is not because of the chapter’s difficulty level, but rather it is due to the student’s inept approach in preparing the material. It is always a good idea to plan ahead of time and examine the substance of the chapter. This not only produces momentum, but also gives a preview of the full chapter, and this structured type of concept creation will lead to better concept formulation and exam acing. Chemical kinetics is one such chapter in chemistry class XII that will be discussed here. Before diving into this obvious chapter, we’ll go through the contents, important subjects, methodology, and prerequisites. Many times, a student’s plan for preparing this chapter consists on memorizing equations while ignoring the conceptual part, resulting in superficial knowledge that is difficult to maintain in the long run. Chemical kinetics is not only one of the most interesting chapters to prepare in class XII, but it is also quite important because the other two chapters, chemical equilibrium and electrochemistry, are fully dependent on chemical kinetics. The rate law of chemical reaction is the subject of the entire chapter of chemical kinetics. Understanding this one law is critical to get high grades in this chapter. Every year, at least one one-mark question is asked in the JEE mains and NEET exams. But, most importantly, this chapter serves as a basis for the other two chapters on the line, chemical equilibrium and electrochemistry. After you’ve finished the NCERT for this chapter, you should read OP Tandon’s book.
Before diving into the study material of this chapter, it is important to first grasp what chemical kinetics is all about. Chemical kinetics is concerned with determining the average rate of reaction and the instantaneous rate of reaction, as well as the numerous factors that can influence these rates. The following are some of the basic prerequisites for preparing this chapter:
The change in concentration of a reactant or product per unit time is defined as the rate of chemical reaction. Consider a hypothetical response from point A to point B.
read more : topworldzone
Rate of disappearance of A= Decrease in concentration of A/Time taken=ΔA/Δt
Rate of disappearance of B= Increase in concentration of B/time taken= ΔB/Δt
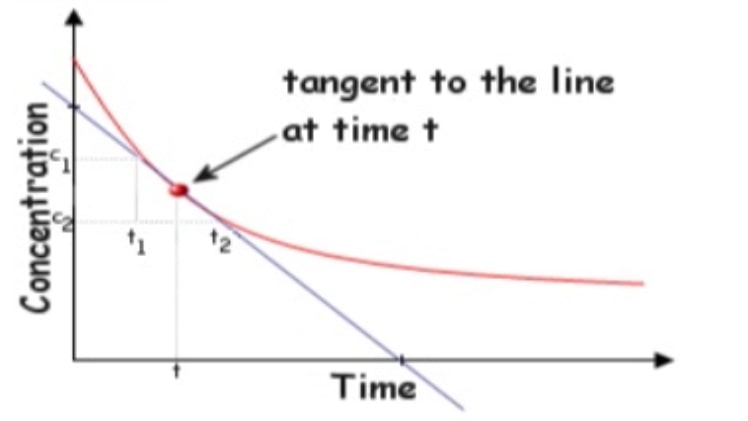
Instantaneous rate of reaction: It is the slope of tangent at time t on concentration time curve.
Average rate of a reaction: It is when there is a change in the concentration of reactants or products and how long it takes for that change to happen, which is called change in concentration.
Factors that influence the rate at which a reaction occurs
- Concentration: The higher the concentration of reactants at a particular temperature, the faster the reaction.
- Temperature: As the temperature rises, so does the rate of chemical reaction.
- Catalyst: When a catalyst is added to a reaction, the rate of the reaction rises.visit here to know more information : topworld45
Rate Constant and Rate Expression
Assume a broad reaction to the situation at hand.
aA + bB -> cC + dD where a,b,c and d are the stoichiometric coefficients
Rate expression can be given as:
Rate α [A]x[B]y
dR/dt=k[A]x[B]y ; The stoichiometric coefficients may or may not be equal to x and y. Instantaneous rate can be expressed as the rate at which a reactant vanishes or as the rate at which a reactant appears.
This implies that
Here k=rate constant and x and y are the partial order with respect to A and B respectively.
The sequence of a reaction
- The overall order of a reaction is given by the sum of the exponents, x+y, in the rate equation.
- A reaction’s order can be integer or fractional.
- A zero order reaction is one in which the rate of reaction is unaffected by reactant concentration.
- For different orders of reactions, a unit of rate constant can be determined as follows:
- The term “elementary reaction” refers to a reaction that occurs in a single step.
- A complicated reaction is one that cannot be completed in a single step but rather requires a series of steps.
Finally, the following are the most essential subjects in chemical kinetics:
- Integrated Rate Equations (IREs) are a type of rate equation that is used to
- The rate at which a chemical reaction occurs.
- The Collision Theory of Chemical Reactions is a theory that describes how chemical reactions collide.
- Factors which influence the rate at which a reaction happens
- The reaction is a pseudo-first order reaction.
- The rate of a reaction is temperature dependent.
Check this important question:
Periodic revision is a crucial part of every chapter in chemistry in order to achieve a high grade. We humans are always prone to forgetting things if we don’t see them on a regular basis. Chemical equations, chemical reactions, and chemical structures of numerous chemical elements are all easily forgotten by pupils. It is highly advised that students keep a notepad for this chapter in which they can keep track of anything they might forget.
Visit here : onlinewebworld24





